Remember “leaded” gasoline? If you don’t you might not even think to wonder why gas is called UN-leaded these days. Lead, which if you haven’t heard, is bad for you. Really bad. And it isn’t good for other living things either, or for you to eat those other-living-things. Lead was used and still is used for many industrial purposes, just ask the Chinese as it seems they put it everything they ship back to us ‘mericans. Lead was phased out in all on-road cars and trucks by 1996 in the United States.
So what was it used for and why was it in gasoline? Lead is really good at raising the octane rating of fuel and extending the life of valves, guides, and seats. The lead that was used in gasoline wasn’t just chunks of metal, it was introduced to gas as tetraethyl lead, soluble in gas. Leaded gas was the best, most economical way to boost octane not only because the chemical itself was inexpensive, but gas would last much longer than today’s blends of alcohols and is much more stable.
With modern fuel injection and hardened valves and seats, lead is not needed in passenger cars. Tetraethyl lead is still used in aircraft that have piston engines and some farm equipment ( ironic since farms are THE source of food almost all Americans). Besides the fact that lead is bad for you and other living things, it is also bad for catalytic convertors. Right about the same time the California Air Resource Board was figuring out that lead was bad for people and other living things, they noticed that the catalytic convertors that had been installed to combat smog were failing. Which is 2 nails in the coffin for lead: pollution, and the killing of pollution-fighting car parts…
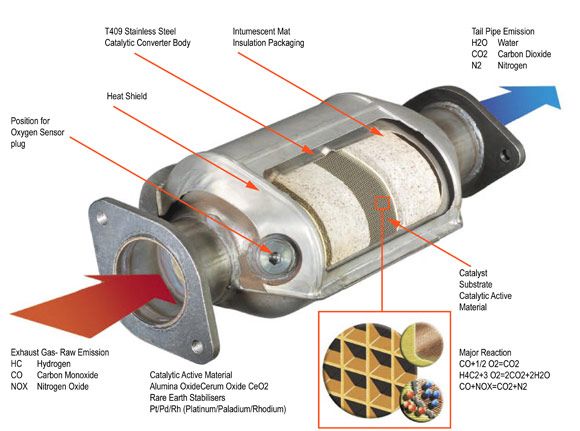
Since 1975, every passenger car and truck has been sold in California with a catalytic convertor. Essentially a furnace, they burn off and oxidize the unused fuel and oil vapors that would normally be spewed out of the tail pipe into the atmosphere. A catalytic convertor uses different compounds to store and then burn oxygen with the contaminants. In the early days of cats they were simple and did not need to reduce nitrogen oxides, and since fuel injection was rare and imprecise anyway, there needed to be an air injection system to help bring oxygen into the catalyst. They were also very restrictive and reduced the power an engine could make, like trying to run around the block while breathing through a straw. The old style 2-way cats were also prone to burning since fuel metering usually erred on the rich side to prevent engine damage. As the CARB standards for pollution on cars and trucks sold in California steadily became more strict, and required vehicle emissions to comply with oxides of nitrogen reduction, the old 2-way catalytic convertors were phased out in favor of 3-way catalytic convertors.
Unlike a 2-way cat, a 3 way catalytic convertor must reduce oxides of nitrogen. A 2-way cat only has to burn off hydro carbons, and oxidize carbon monoxide. It isn’t that chemically hard. You add oxygen and heat. Done. But, a 3 way has to do that, and REDUCE nitrogen oxides. That means taking AWAY oxygen for one reaction and adding it for the other two. At the same time. That would be like burning a pile of wood and trying to pull fresh leaves out of the smoke.
Well, it turns out that it isn’t impossible to do all three, just a little tricky, and expensive. To reduce oxides of nitrogen (NOx) the fuel injection needs to burn rich (more gas than air) and the best way to burn off hydro carbons (HC) is to burn lean (more air than gas). Carbon monoxide (CO) is turned into carbon dioxide (CO2) when exposed to additional oxygen as well. So the fuel injection computer will cycle between rich and lean to keep all 3 reactions going. That’s the tricky (and actually much more complicated than this simple explanation). The expensive is the use of precious metals to store and reduce oxygen. Platinum is used as a reducer as well as oxidizer, but there is also palladium and Rhodium, as well as Cerrium, Iron, Manganese, and Nickel. The exact formulation will vary from vehicle and make.
Fuel injected vehicles have come a long way in metering air and fuel to achieve perfect or stoichiometric combustion. This point is between 14.6 and 14.8 parts air to 1 part fuel, by weight, for gasoline. In other words, it takes a lot of air to burn a little bit of gas. But air isn’t just oxygen, it is a lot nitrogen, mostly nitrogen in fact. That means there isn’t just the gas and all of the added detergents and octane boosters, but all the nitrogen in the air we breathe and burn in our cars which is why it is hard to remove the NOx. Since 1996 and the standardization of OBDII (OnBoard Diagnostics version2), the dreaded P0420 has become an expensive Check Engine Light code.
P0420 is a code stored in the fuel injection computer when it detects the catalytic convertor has deteriorated its ability to oxidize Hc and CO. The way the computer does this is by monitoring the content of oxygen in the exhaust. If the Oxygen Sensor downstream from the cat is highly reactive or shows a cycle of lean to rich, similar to the front oxygen sensor, the cat is deemed bad and the code is set. Each vehicle make uses a different criteria and standard for setting the code, as some use Air/Fuel sensors instead or with oxygen sensors ( a topic for another time). As of 2012 there is no code for a deterioration in reducing NOx.
So what causes the cat to stop working like it should? In some instances a cat gets used up, in other instances it is because of contamination. Lead was phased out of gasoline because it coated the substrate in the cat and stopped oxidation from happening.
Another example of contamination is when the engine is burning oil excessively or engine coolant from a bad head gasket. The additives in oil and the silicon and phosphorus in coolant are the chemicals that contaminate the substrate in the cat. Zinc used to be used in older engine oil formulas to help prevent the wear of internal engine parts like camshafts and crankshafts and it, like lead, was cheap and very effective, but also like lead, coated the substrate in the cat and stopped oxidation.
It is worth mentioning that if you have an older (around 1990) car you should look for an API rating of “SL” because it has the most zinc and will help prolong the life of your engine. Zinc additive is mandatory for vintage engines like the venerable 2F motor in Fj land cruisers!
Contamination is not the only way catalytic convertors are ruined, engine and fuel injection problems that cause a misfire are actually the most common way for cats to be destroyed. When a cylinder misfire happens unburned, raw fuel enters the exhaust stream and is then burned in the catalytic convertor. When this happens the cat will overheat and can actually melt the ceramic substrate. When the cat is melted or cracked from a misfire, the exhaust can become plugged reducing engine power.

Catalytic convertors are one of the most expensive parts of the emissions system and are usually covered under a federal warranty of 80,000 miles, regardless of vehicle make, on cars and trucks from 1996 and newer. Of course if it is damaged from a misfire you are out of luck. If you have a misfire bad enough to damage the catalytic convertor the check engine light will blink or flash to warn you. This is true for ALL passenger cars and trucks made in 1995 and newer, and is mentioned in every owner’s manual; you can’t plead ignorance when trying to warranty this type of damage..
The code P0420 is not always caused by a worn out or inefficient catalyst. Some late model cars and trucks suffered from bad software in the fuel injection computer that either damaged the cat, or the software was not calibrated correctly to recognize that the cat is actually doing its job. Lots of toyota matrix, corolla, tundra, sequoias, and 4Runners have had to have their computers re-flashed with updated software. This should be covered under the federal warranty (80,000 miles or 96 months). Of course Toyota is not the only make that has software updates, I think just about every single make has at least one model that could use a re-flash before replacing the convertor.
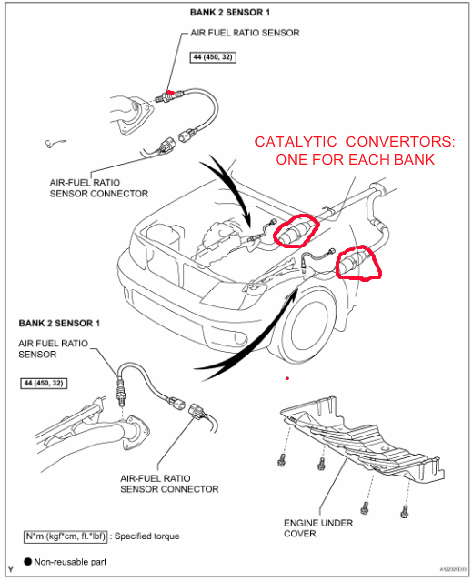
So how expensive is a catalytic convertor? It depends. Typically the newer the vehicle the more strict emissions compliance is, so that cat needs to be doing a good job oxidizing/reducing, which requires more design expense, materials cost and manufacturing precision. Late model vehicles typically cost at least $900 all the way up to $1500 or more. For one cat. If you have a V6 or V8 you will have at least 2 catalytic convertors- one for each bank or side of the engine.
More and more vehicles are using Pre-Cats in the exhaust system. This is a cat that is not usually monitored by the fuel injection computer. It is located as close to the exhaust output as possible, sometimes it is integral to the exhaust manifold. The fuel injection computer will retard igntion timing during a cold start to heat up the Pre-Cat to get the exhaust hot enough to “light off” the primary catalyst down stream. These guys, even though they are not usually monitored, can cause the P0420 if they are cracked or if they do not keep the exhaust hot enough wile the fuel injection computer is monitoring the downstream cat. These also can be several hundred dollars in parts cost alone, not to mention the labor cost.
As some people have been painfully aware, thieves sometimes cut the convertor out in hopes of taking it to a metal recycle facility for quick cash. The amount the recycler pays out is nothing compared to the cost of replacing a modern catalytic convertor. I have read in the news anywhere from $30 up to $200, but when it costs thousands to replace them it hits the wallet pretty hard when you start your truck and it sounds like a flat track racer. Thieves suck. Around Santa Cruz and the bay area, Toyota trucks and SUVs are the target of choice since the cat is positioned in an easy to get to location and are quickly cut off with a pipe cutter tool or saw. There is no good way to protect the cats on these vehicles other than fabricating a big heavy skid-plate that would mount to the bottom of the truck. Hopefully laws will be passed soon requiring identification and a VIN number for cat recycling.
For all the good that catalytic convertors do at reducing emissions, it is ironic to me that for them to work their absolute best, a car or truck has to be running its best or as close to stoichiometric as possible. Theoretically, when gas is burned in an engine, the only things coming out the tail pipe are water and carbon dioxide(with a small amount of NOx), our 2 favorite green house gasses. It will be interesting to see how these 2 gasses are dealt with in the coming years. Strict electric? Hydrogen Fuel Cells? Bikes? Horse and Carriage?